Abstract
Central nervous system (CNS) relapse is a principal cause of treatment failure among pediatric patients with acute lymphoblastic leukemia (ALL). Furthermore, available CNS-directed therapies are associated with significant short and long-term morbidities. While extensive research has demonstrated a critical role of the bone marrow microenvironment in leukemia biology, how the CNS regulates ALL survival and chemoresistance has yet to be fully determined.
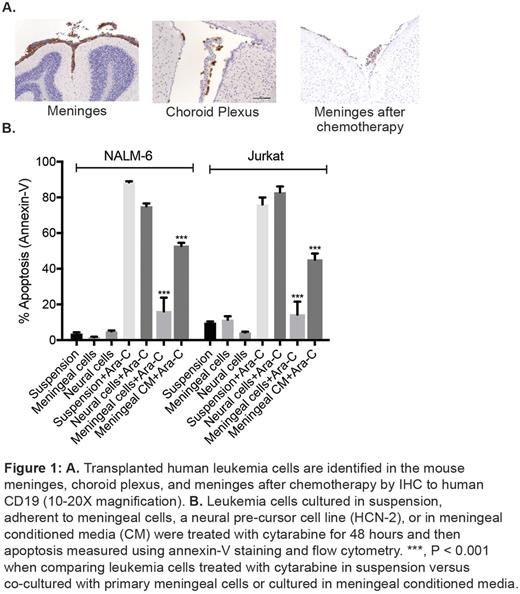
To identify where leukemia cells home within the CNS, we transplanted multiple human leukemia cell lines into immune-compromised mice. After systemic leukemia development that included the CNS, the mice were euthanized, fixed, and examined by histopathology and immunohistochemistry. We identified both the meninges and, to a lesser extent, the choroid plexus as the predominant CNS sites that harbor leukemia cells both before and after treatment with systemic cytarabine (Figure 1A).In contrast, parenchymal involvement by leukemia was a rare, and often late, finding.This pattern of CNS involvement by leukemia also recapitulates the histopathology of human CNS leukemia.
We then used ex vivo co-culture approaches to focus more specifically on the effects of the meninges on leukemia biology. We found that leukemia cells adhered to meningeal cells in a co-culture system that can substitute human cerebral spinal fluid for tissue culture media. Moreover, co-cultured leukemia cells were significantly more resistant to cytarabine-induced apoptosis, as measured by annexin-V staining, relative to the same leukemia cells grown in suspension or adherent to either fibronectin-treated plates or a neural precursor cell line (Figure 1B). Extending these results, meningeal-mediated leukemia chemoresistance was also observed with methotrexate as well as when measured by caspase 3/7 & 8 activity and mitochondrial polarization by TMRE staining. Moreover, primary ALL cells exhibited significantly increased apoptosis when cultured in suspension relative to when co-cultured with primary meningeal cells (60.4% vs. 8.9%, P <0.0001), further supporting the pro-survival effect of meningeal cells on leukemia cells. Finally, while leukemia cells co-cultured with meningeal cells exhibited striking chemoresistance, leukemia cells cultured in meningeal conditioned media also exhibited moderate chemoresistance (Figure 1B). Together, these results suggest that both a mechanism dependent upon direct cell contact and a soluble factor(s) secreted by meningeal cells contribute to meningeal-mediated leukemia chemoresistance.
In order to better understand the mechanism of meningeal-mediated leukemia chemoresistance, we examined the effect of co-culture on the apoptosis pathway in leukemia cells. An apoptosis antibodyarray showed changes in the expression of multiple apoptosis family proteins (including caspase 3, caspase 8, Bax, Bad, BID, BIM, BCL-2, SMAC and survivin) in leukemia cells co-cultured with meningeal cells relative to leukemia cells in suspension. However, assessing how the dynamic levels, activities, and complex interactions of these and other BCL-2 family of pro-and anti-apoptotic proteins (BH3 proteins) integrate to regulate the overall apoptotic balance is experimentally challenging. To address this issue, we utilized a functional apoptosis assay, BH3 profiling, which predicts cellular responses to stimuli based on measuring the response of mitochondria to perturbation by a panel of BH3 domain peptides. BH3 profiling of leukemia cells in suspension or co-cultured with meningeal cells showed that leukemia cells co-cultured with meningeal cells are significantly less primed to undergo apoptosis relative to leukemia cells in suspension.
In summary, these results show that the meninges provide a unique CNS leukemia niche that regulates the apoptotic balance in leukemia cells in order to enhance leukemia chemoresistance and contribute to relapse. This work also suggests that CNS leukemia persistence and relapse is not only related to poor penetration of some chemotherapy agents across the blood-brain barrier and into the CNS. Further characterization of the cellular and molecular mechanisms of meningeal-mediated leukemia chemoresistance will provide new insights into the pathophysiology of CNS leukemia as well as inform novel therapeutic strategies to target leukemia cells in the CNS niche.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal